King's Health Partners Clinical Trials Office
Advancing high-quality clinical research through collaboration, expertise, and world-class facilities across King’s Health Partners.

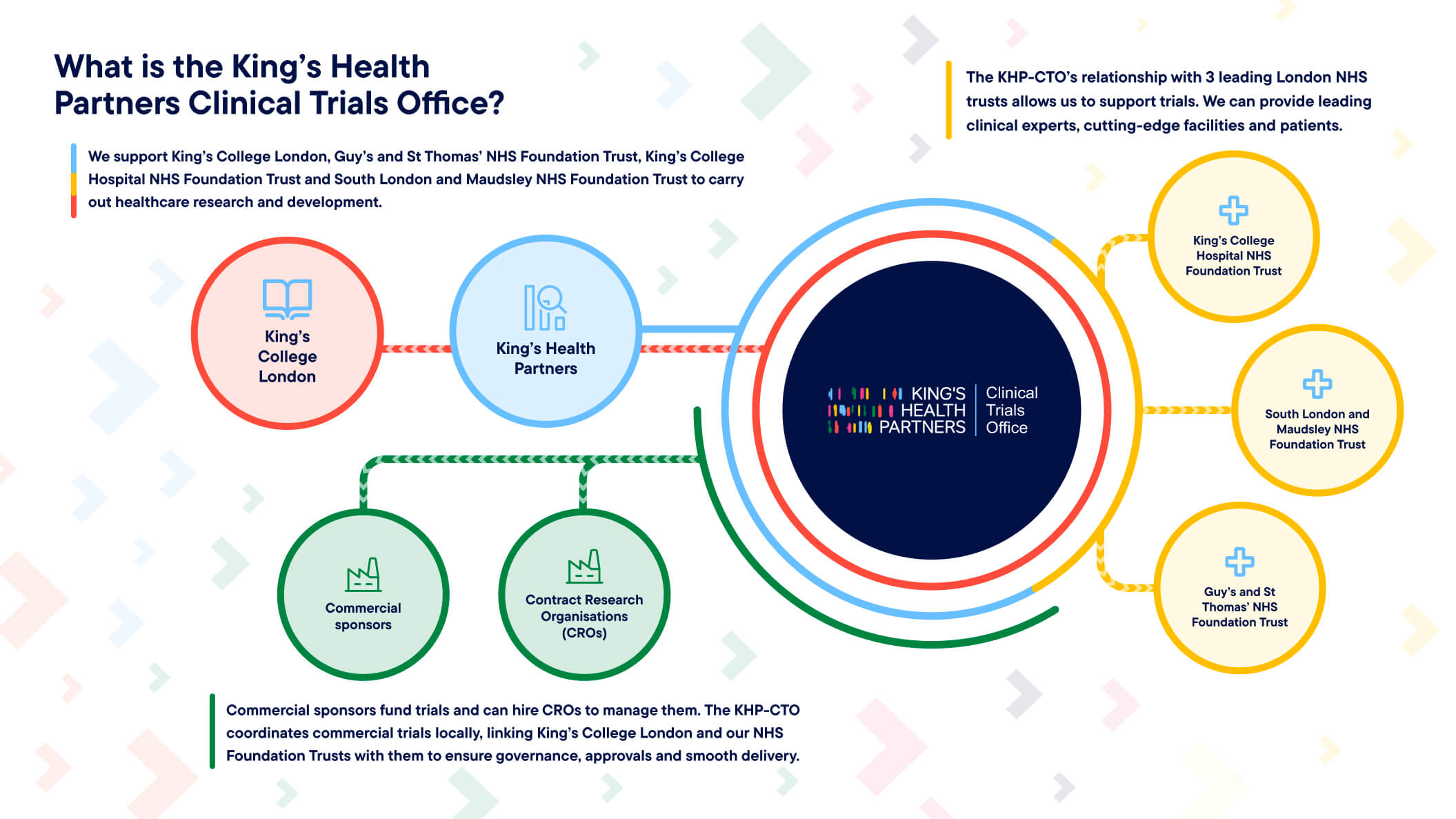
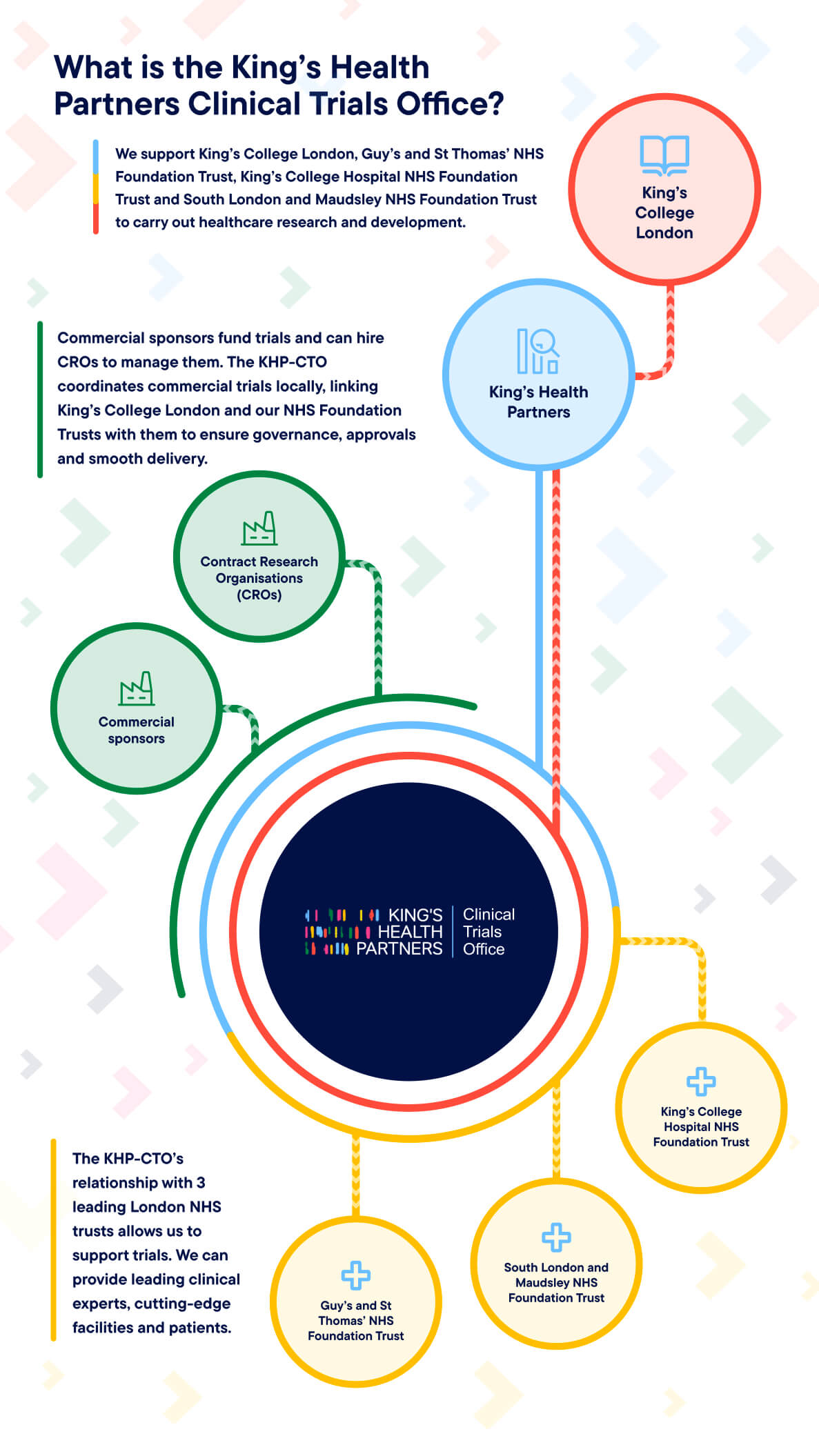
What is the King's Health Partners Clinical Trials Office?
King’s Health Partners Clinical Trials Office (KHP-CTO) brings together the globally renowned university King’s College London and three leading NHS Foundation Trusts to deliver high-quality clinical trials that drive better care.
KHP-CTO is an established joint initiative between:
- King’s College London (King’s)
- Guy’s and St Thomas’ NHS Foundation Trust (GSTFT).
- King’s College Hospital NHS Foundation Trust (KCH).
- South London and Maudsley NHS Foundation Trust (SLaM).
KHP-CTO operates as an extension of the Research and Development departments at three sovereign organisations: Guy’s and St Thomas’, South London and Maudsley and King’s College Hospital Foundation Trusts.
How can King's Health Partners Clinical Trials Office support my clinical trial?
Working with our partners, we can provide:
- World-class clinical and research facilities.
- Access to a large and diverse patient population across one mental health and two acute NHS Foundation Trusts.
- Academic and NHS researchers working in partnership.
We support a wide range of industry-sponsored and investigator-led studies across mental health, acute care and other settings. KHP-CTO offers a seamless interface between academia, the NHS and life sciences partners. We provide world-class academic insight, clinical excellence, and a diverse patient population to both researchers and commercial sponsors and CROs.
Our Commercial Team delivers a single point of contact for industry-sponsored clinical trials. Our Non Commercial Team ensures regulatory compliance and smooth delivery of sponsor obligations within our research community. Our Training Team delivers Good Clinical Practice (GCP) training across all partner organisations to maintain the highest research standards.
Our Non-Commercial Team
Our team of Clinical Research Associates (CRAs), Clinical Trial Administrators (CTAs) and dedicated trainers uphold the highest standards in clinical trials.
We operate under a robust set of Standard Operating Procedures (SOPs) and policies, ensuring full compliance with Good Clinical Practice (GCP) and regulatory requirements. Our CRA team partner closely with investigator teams, conducting regular monitoring visits to maintain quality and integrity at every trial site.

Our Commercial Team
Delivering a streamlined, expert-led service for commercial sponsors and investigators, our Commercial Team simplifies the setup and management of clinical trials across our partner institutions, ensuring compliant, consistent, efficient processes from start to finish.
With integrated clinical quality management and training functions, we uphold high standards at every site. Our skilled and knowledgeable research staff conduct trials in line with the latest best practices, driving excellence in commercial clinical research.

Our Training
Our courses are free for staff and students at King’s College London, King’s College Hospital, South London and Maudsley, and Guy’s and St Thomas’ NHS Foundation Trusts.
Whether you’re new to clinical research or want to refresh your expertise, our training equips you with the skills and knowledge to conduct high-quality, compliant clinical trials with confidence

Frequently asked questions
More information about our work and the support we provide.
Please submit an Expression of Interest (EOI) request via the NIHR Site Identification Service.
No. Health Partners Clinical Trials Office (KHP-CTO) is part of King’s College London. While King’s does not have a direct relationship with patients, our relationship within KHP means commercial researchers can have access to up to 3.7 million patients our partner trusts care for each year.
The national target is 150-calendar days from HRA submission to confirmation of Capacity and Capability (C&C).
If the Sponsor is a for-profit organisation like a pharmaceutical company, the study is commercial. If the Sponsor is a non-profit organisation like a public hospital or university, the study is non-commercial.
The KHP-CTO aims to help commercial Sponsors/CROs open studies at its Partner Trusts as fast as possible.
The KHP-CTO helps Partner Trusts set up non-commercial CTIMPs, sponsored or co-sponsored by the Partner Trusts, in collaboration with the Partner Trusts’ Research and Development departments. Once open, we can provide monitoring across all European sites (not just the Partner Trust’s sites).
Yes. The Research Teams at our Partner Trusts can work with external services (e.g. home nursing), provided that the roles and responsibilities for all protocol-related activities are clearly formalised in a signed Communication Plan.
Materials can be shipped to a Partner Trust at any time, but the Trust cannot take responsibility for such items until the study contract is signed.
Please email your CDA enquiries to King’s Health Partners Clinical Trials Office Commercial Team.
No. KHP-CTO is a directorate within King’s College London (KCL), one of the UK’s leading universities. Since 2001, three hospital groups, also known as our Partner Trusts (Guy’s and St Thomas’ NHS Foundation Trust, King’s College Hospital NHS Foundation Trust, and South London and Maudsley NHS Foundation Trust) have paid KHP-CTO to represent them in respect of commercial research, and to provide specialist support for the non-commercial CTIMPs they run.